Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Grund, J.*; Asai, Masato; Blaum, K.*; Block, M.*; Chenmarev, S.*; D llmann, Ch. E.*; Eberhardt, K.*; Lohse, S.*; Nagame, Yuichiro*; Nagy, Sz.*; et al.
llmann, Ch. E.*; Eberhardt, K.*; Lohse, S.*; Nagame, Yuichiro*; Nagy, Sz.*; et al.
Nuclear Instruments and Methods in Physics Research A, 972, p.164013_1 - 164013_8, 2020/08
Times Cited Count:6 Percentile:59.94(Instruments & Instrumentation)We report on the successful coupling of the Penning-trap mass spectrometry setup TRIGA-TRAP to the research reactor TRIGA Mainz. This offers the possibility to perform direct high-precision mass measurements of short-lived nuclei produced in neutron-induced fission of a  U target located near the reactor core. An aerosol-based gas-jet system is used for efficient transport of short-lived neutron-rich nuclei from the target chamber to a surface ion source. In conjunction with new ion optics and extended beam monitoring capabilities, the experimental setup has been fully commissioned. The design of the surface ion source, efficiency studies and first results are presented.
U target located near the reactor core. An aerosol-based gas-jet system is used for efficient transport of short-lived neutron-rich nuclei from the target chamber to a surface ion source. In conjunction with new ion optics and extended beam monitoring capabilities, the experimental setup has been fully commissioned. The design of the surface ion source, efficiency studies and first results are presented.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Beerwerth, R.*; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina*; Nagame, Yuichiro; Osa, Akihiko; Toyoshima, Atsushi; et al.
Journal of the American Chemical Society, 140(44), p.14609 - 14613, 2018/11
Times Cited Count:29 Percentile:69.01(Chemistry, Multidisciplinary)The first ionization potential (IP ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Sato, Tetsuya
Genshikaku Kenkyu, 61(1), p.96 - 106, 2016/09
We successfully determined the first ionization potential of lawrencium (Lr, Z=103). The result experimentally substantiated for the first time that Lr is the last member of the actinide series. Measured ionization potential suggested that Lr atom would have the electronic configuration which is different from the configuration expected based on the Periodic table. For the measurement, we have developed a novel method applied the surface ionization process. Public responses after the publication are also introduced.
Sato, Tetsuya
Isotope News, (740), p.16 - 19, 2015/12
We successfully determined the first ionization potential of lawrencium (Lr, Z=103). The result experimentally substantiated for the first time that Lr is the last member of the actinide series. Measured ionization potential suggested that Lr atom would have the electronic configuration which is different from the configuration expected based on the Periodic table.
Sato, Tetsuya
Nihon Genshiryoku Gakkai-Shi ATOMO , 57(11), p.741 - 744, 2015/11
, 57(11), p.741 - 744, 2015/11
We have experimentally confirmed that Lr would be the last member of actinides series for the first time by a measurement of the first ionization potential of lawrencium (Lr, element 103). The electronic orbital of Lr atom which is estimated by the result suggests that Lr could have the outermost electronic orbital similar with group-13 elements. This work triggered a discussion concerning positions of Lr and lutetium, lanthanide homologue of Lr.
Sato, Tetsuya
Hosha Kagaku, (32), p.34 - 41, 2015/09
In the surface ionization process, an ionization efficiency depends on the first ionization potential of the atom of the element. The ionization potential can be estimated by using the relationship. This method has been developed in order to determine the first ionization potential of lawrencium (Lr, element 103). The value of the ionization potential of Lr have not been measured experimentally due to its low production rate and short half-life. The surface-ionization method is described in detail in this paper.
Sato, Tetsuya; Nagame, Yuichiro; Tsukada, Kazuaki
Kagaku To Kogyo, 68(9), p.824 - 826, 2015/09
We successfully confirmed that lawrencium (Lr, element 103) is the last member of actinide series by a measurement of its first ionization potential. Obtained experimental result suggested that the outermost electronic orbital of Lr atom would have p-orbital similar to elements of group-13. Our result triggered again the discussion of the position of Lr and lutetium, the lanthanide homologue of Lr, on the Periodic Table.
 -decays of neutron-deficient americium isotopes
-decays of neutron-deficient americium isotopesSakama, Minoru*; Asai, Masato; Tsukada, Kazuaki; Ichikawa, Shinichi; Nishinaka, Ichiro; Nagame, Yuichiro; Haba, Hiromitsu*; Goto, Shinichi*; Shibata, Michihiro*; Kawade, Kiyoshi*; et al.
Physical Review C, 69(1), p.014308_1 - 014308_11, 2004/01
Times Cited Count:22 Percentile:74.84(Physics, Nuclear)no abstracts in English
 aerosol jet system for mass separation of neutron-deficient actinide isotopes
aerosol jet system for mass separation of neutron-deficient actinide isotopesIchikawa, Shinichi; Tsukada, Kazuaki; Asai, Masato; Haba, Hiromitsu; Sakama, Minoru*; Kojima, Yasuaki*; Shibata, Michihiro*; Nagame, Yuichiro; Oura, Yasuji*; Kawade, Kiyoshi*
Nuclear Instruments and Methods in Physics Research B, 187(4), p.548 - 554, 2002/04
Times Cited Count:15 Percentile:65.5(Instruments & Instrumentation)no abstracts in English
Osa, Akihiko; Koizumi, Mitsuo; Sekine, Toshiaki; Katsuragawa, H.*; Jin, W.*; Wakui, Takashi*
JAERI-Review 99-025, TIARA Annual Report 1998, p.203 - 205, 1999/10
no abstracts in English
 La isotopes
La isotopesYamamoto, Hiroshi*; Shibata, Michihiro*; Kawade, Kiyoshi*; Kojima, Yasuaki*; Osa, Akihiko; Watanabe, Satoshi; Koizumi, Mitsuo; Sekine, Toshiaki
JAERI-Review 99-025, TIARA Annual Report 1998, p.208 - 210, 1999/10
no abstracts in English
 measurements of
measurements of  La
LaKojima, Yasuaki*; Asai, Masato*; Osa, Akihiko; Koizumi, Mitsuo; Sekine, Toshiaki; Shibata, M.*; *; Kawade, Kiyoshi*; Tachibana, Takahiro*
Journal of the Physical Society of Japan, 67(10), p.3405 - 3413, 1998/10
Times Cited Count:10 Percentile:58.58(Physics, Multidisciplinary)no abstracts in English
 Sm and
Sm and 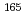 Gd
GdIchikawa, Shinichi; Tsukada, Kazuaki; Nishinaka, Ichiro; Iimura, Hideki; ; Nagame, Yuichiro; Asai, Masato*; Kojima, Yasuaki*; *; Shibata, M.*; et al.
Physical Review C, 58(2), p.1329 - 1332, 1998/08
Times Cited Count:13 Percentile:60.06(Physics, Nuclear)no abstracts in English
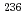 Am
AmTsukada, Kazuaki; Ichikawa, Shinichi; Hatsukawa, Yuichi; Nishinaka, Ichiro; ; Nagame, Yuichiro; Oura, Yasutsugu*; *; Sueki, Keisuke*; *; et al.
Physical Review C, 57(4), p.2057 - 2060, 1998/04
Times Cited Count:15 Percentile:63.99(Physics, Nuclear)no abstracts in English
Ichikawa, Shinichi
Bunseki Kagaku, 47(7), p.461 - 462, 1998/00
no abstracts in English
Ichikawa, Shinichi
KURRI-KR-25, p.13 - 15, 1998/00
no abstracts in English
Osa, Akihiko; Koizumi, Mitsuo; Sekine, Toshiaki; Yamamoto, Hiroshi*; *; *; *
"Fuanteikaku No Rikogaku Oyobi Kaku Keisokuho(II)" Ni Kansuru Semmon Kenkyukai Hokokusho, 0, p.28 - 30, 1997/00
no abstracts in English
; Tsukada, Kazuaki; Asai, Masato*; Osa, Akihiko; Oura, Yasutsugu*; *; *; Nishinaka, Ichiro; ; Nagame, Yuichiro; et al.
Nuclear Instruments and Methods in Physics Research B, 126(1-4), p.205 - 208, 1997/00
Times Cited Count:5 Percentile:46.15(Instruments & Instrumentation)no abstracts in English
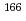 Tb
TbAsai, Masato*; Tsukada, Kazuaki; ; Osa, Akihiko; *; *; Yamamoto, Hiroshi*; Kawade, Kiyoshi*; Shinohara, Nobuo; Nagame, Yuichiro; et al.
Journal of the Physical Society of Japan, 65(5), p.1135 - 1138, 1996/05
Times Cited Count:13 Percentile:67.83(Physics, Multidisciplinary)no abstracts in English
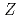 = 102)
= 102)Sato, Tetsuya; Asai, Masato; Kaneya, Yusuke; Tsukada, Kazuaki; Toyoshima, Atsushi; Takeda, Shinsaku; Mitsukai, Akina*; Nagame, Yuichiro; Ichikawa, Shinichi; Makii, Hiroyuki; et al.
no journal, ,
We successfully determined the first ionization energy (IE) of nobelium (No, 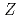 = 102) using a short-lived No isotope,
= 102) using a short-lived No isotope, 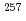 No produced in the
No produced in the Cm(
Cm( C, 4n) reaction, based on the IE dependence of the ionization efficiency in a surface ionization process. The IE value of No was evaluated to be 6.6 eV. This value is in a good agreement with the value which has been estimated by an extrapolation from those of the lighter actinide elements, 6.65 eV.
C, 4n) reaction, based on the IE dependence of the ionization efficiency in a surface ionization process. The IE value of No was evaluated to be 6.6 eV. This value is in a good agreement with the value which has been estimated by an extrapolation from those of the lighter actinide elements, 6.65 eV.